Why the Membrane Matters More Than the Fabric in Viral Protection

In the high-stakes environment of medical infection control, the true engine of safety is not the visible textile, but the "invisible" functional membrane hidden within. While the outer fabric provides mechanical properties like tear resistance and comfort, it is the 0.01 mm functional membrane that serves as the actual barrier against microscopic pathogens. By engineering this layer with zero-tolerance precision, technologies like MediShield™ achieve the gold standard of AAMI Level 4 and ASTM F1671 viral protection. This article explores why the membrane's polymer science and manufacturing uniformity are the most critical factors in safeguarding healthcare professionals, and how Kae Hwa Industrial transforms micrometer-level precision into a world of global trust.
The Engine of Protection: Why the Membrane is the Actual Barrier
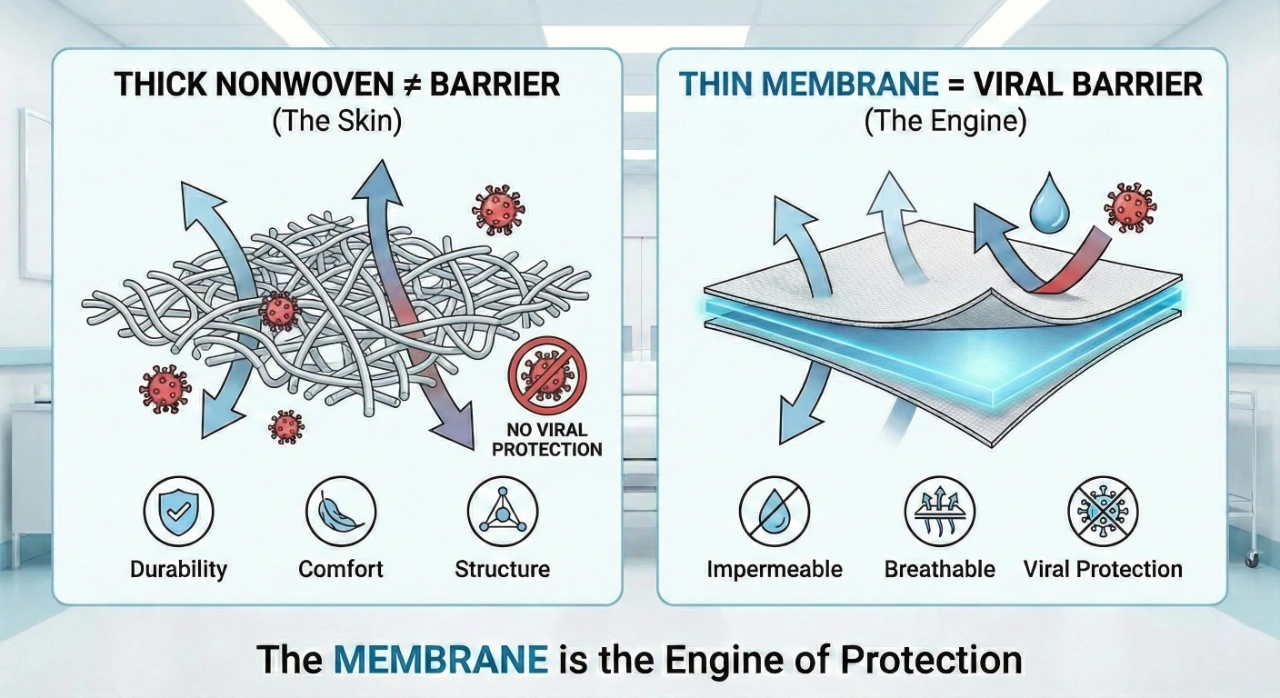
In the world of medical procurement, a common misconception often leads decision-makers astray: the belief that a thicker, heavier fabric inherently provides superior protection. However, in advanced material science, the outer fabric (the "skin") and the internal membrane (the "engine") serve two entirely different purposes.
The fabric layer is designed for:
- Mechanical Durability: Protecting the garment from physical snags, tears, and abrasion.
- User Comfort: Providing a soft, textile-like feel against the skin.
- Structure: Giving the medical gown its shape and fit.
In contrast, the viral protection is almost entirely provided by the functional membrane core. This ultra-thin layer is the component that actually manages the delicate balance between total liquid impermeability and high moisture vapor transmission (breathability). Without a high-performance membrane, even the strongest fabric remains porous at a microscopic level, allowing virus-carrying droplets to penetrate. At Kae Hwa, we treat this micrometer-scale barrier as a designed safety distance that keeps danger out while keeping comfort in.
The Science of Precision: Casting Technology and Uniformity
The reliability of a viral barrier is directly linked to the precision of its manufacturing. Even a single microscopic defect or a slight variation in thickness across a roll of film can lead to a catastrophic failure in a high-risk medical setting.
To eliminate these risks, we utilize industry-leading Casting Film Technology. By precisely extruding molten polymer into a thin sheet, we achieve a level of control that is impossible with traditional blown film methods.
Core Advantages of Casting Precision:
- Exceptional Thickness Tolerance: Our automated casting lines maintain a consistent profile across the entire width of the film, typically within a ±0.01 mm margin. This uniformity is essential for downstream lamination and ensures there are no "weak spots" in the final garment.
- Uniform Pore Structure: For microporous films, our process ensures that billions of micro-scale pores are distributed evenly. This leads to predictable Moisture Vapor Transmission Rates (MVTR), ensuring the wearer stays cool without compromising safety.
- Path-Integrity: The casting process creates a smooth, defect-free surface ideal for medical and hygiene applications, where structural integrity is non-negotiable.
MediShield™: Meeting the Gold Standard of AAMI Level 4
To validate technical superiority, a membrane must be tested against the world’s most rigorous infection-control standards. Our MediShield™ series is engineered specifically to meet these requirements, providing a breathable viral barrier that healthcare professionals can trust.
Critical Certifications for Viral Protection:
- AAMI Level 4: The highest standard for surgical gown fluid and viral barrier protection, serving as the benchmark for critical zones in high-risk surgery.
- ASTM F1671: This test measures resistance to penetration by blood-borne pathogens (using a bacteriophage as a surrogate for viruses). Passing this test is the definitive proof of a membrane’s barrier capability.
- EN 14126: The European standard for protective clothing against infective agents, ensuring resistance to pressurized fluids and contaminated aerosols.
These certifications are earned not by the fabric, but by the membrane’s ability to remain liquid-tight and virus-proof even under significant hydrostatic pressure.
Microporous vs. Monolithic: Choosing the Right Protection Mechanism
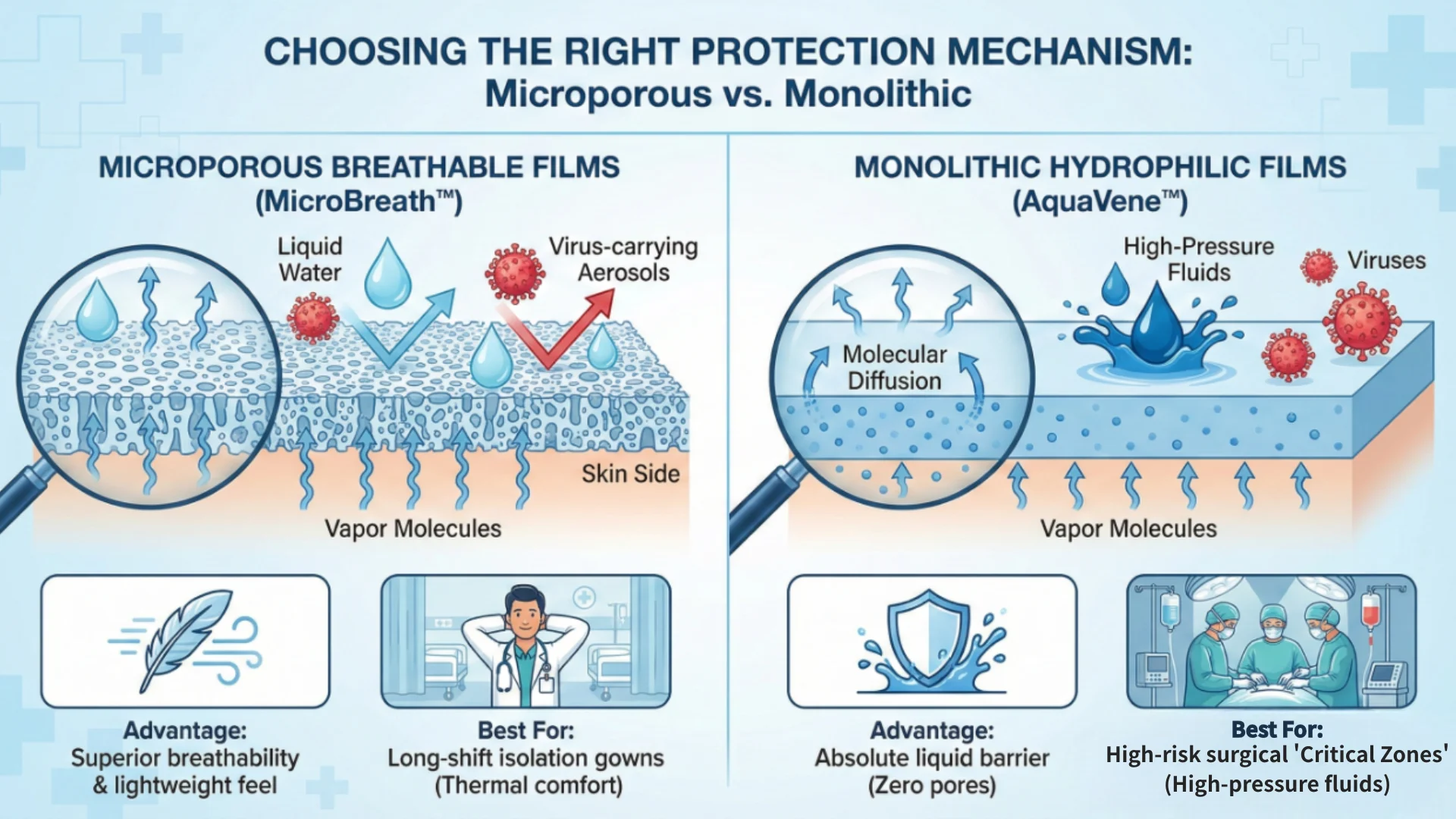
Depending on the medical environment—whether an isolation ward or a high-fluid surgical theater—the type of membrane technology must be strategically selected.
Microporous Breathable Films (MicroBreath™)
These membranes contain billions of micro-scale pores. These pores are large enough for vapor molecules to escape but far too small for liquid water or virus-carrying aerosols to pass through.
- Advantage: Superior breathability and lightweight feel.
- Best For: Long-shift isolation gowns where thermal comfort is a priority.
Monolithic Hydrophilic Films (AquaVene™)
Unlike microporous films, monolithic membranes are solid, non-porous structures. They rely on hydrophilic polymer chemistry where water vapor is absorbed and dispersed through molecular diffusion.
- Advantage: Absolute liquid barrier with zero pores, providing the highest level of isolation.
- Best For: High-risk surgical "Critical Zones" with constant exposure to high-pressure fluids.
Experience as Proof: 80,000 Kilometers of Global Trust
A technical specification is only as good as its real-world performance. Kae Hwa Industrial’s expertise is built on a legacy that began in 1961. Since our commercialization milestone in 2000, where we became the first company in Taiwan to mass-produce breathable PE films, we have focused on one thing: Engineering Invisible Safety.
In 2020, during the global pandemic, our production capabilities were put to the ultimate test. We supplied over 80,000 kilometers of protective fabric to healthcare workers worldwide—enough to circle the earth twice. This experience—managing global supply chains while maintaining zero-defect quality—is why global brands trust Kae Hwa as their partner in breathable protection.
Sustainable Safety: The Future of Functional Membranes
True protection must be sustainable. We believe that safeguarding human life should not come at the cost of the planet. By integrating technology and responsibility, we have redefined the manufacturing of medical laminates.
- Solvent-Free In-Line Lamination: Our system bonds the membrane directly to the fabric immediately after casting, significantly reducing VOC emissions and energy consumption.
- GRS (Global Recycle Standard) Certification: We actively utilize recycled materials in our supply chain, ensuring high-performance fabrics contribute to a circular economy.
- Carbon Footprint Management: Having completed ISO 14064-1 carbon verification, we align our precision engineering with global climate goals.
Elevate Your Protection Strategy: Choose the Engine of Safety
In the medical field, the true measure of safety is the advanced membrane core engineered within your garments. Don't settle for surface-level fabric—ensure your products are powered by the reliability of Kae Hwa’s Casting Film Technology.
- Meet the Gold Standard: Prioritize membranes certified to AAMI Level 4 and ASTM F1671 for maximum viral barrier protection.
- High-Precision Engineering: Benefit from industry-leading manufacturing that ensures zero weak spots in your protective gear.
- A Legacy of Trust: Partner with an expert backed by over 60 years of polymer science and a proven global track record.
Contact Our Specialists for a Technical Consultation
FAQ
Q1. Why is the membrane more important than the fabric in medical gowns?
A1. The fabric provides only mechanical durability and comfort, whereas the membrane is the actual functional layer that blocks microscopic viruses. A precision-engineered membrane ensures that the barrier is uniform and impenetrable to pathogens, which a fabric alone cannot achieve.
Q2. What does AAMI Level 4 certification mean for a membrane?
A2. AAMI Level 4 is the highest protection standard for surgical gowns. It requires the material to pass the ASTM F1671 test, proving it can resist the penetration of blood-borne viruses even under significant fluid pressure.
Q3. How does a waterproof viral barrier remain breathable?
A3. Breathability is achieved through microporous technology (pores large enough for vapor but too small for droplets) or monolithic hydrophilic chemistry (molecular diffusion). This allows heat to escape while keeping the viral barrier intact.
Q4. What is the benefit of Casting Film Technology for medical use?
A4. Casting technology allows for extreme control over film thickness. This ensures a perfectly uniform barrier across the entire garment, eliminating the microscopic weak spots that could lead to viral penetration during medical procedures.